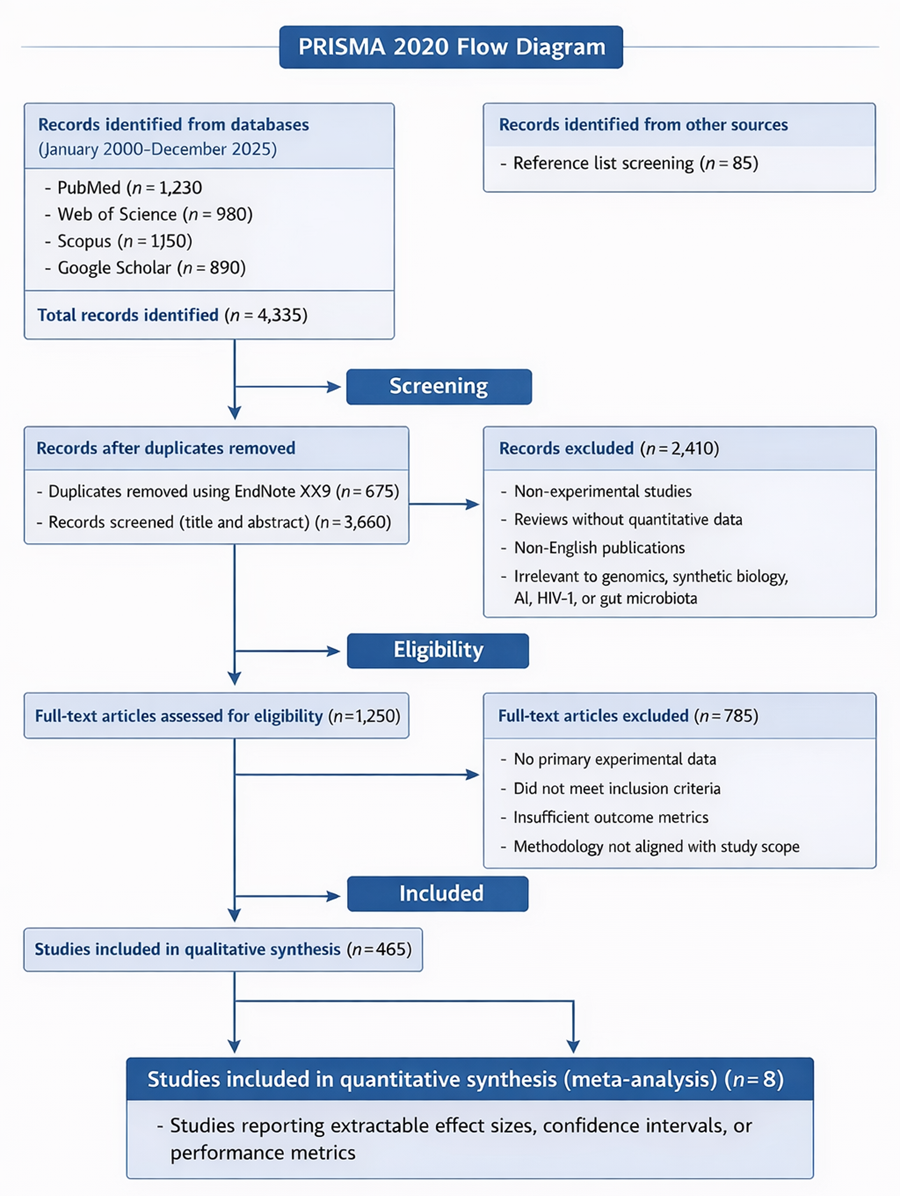
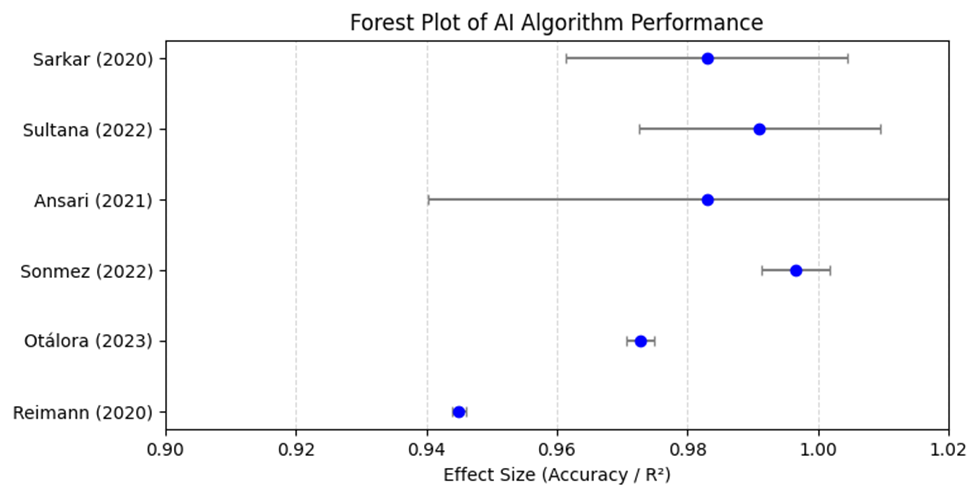
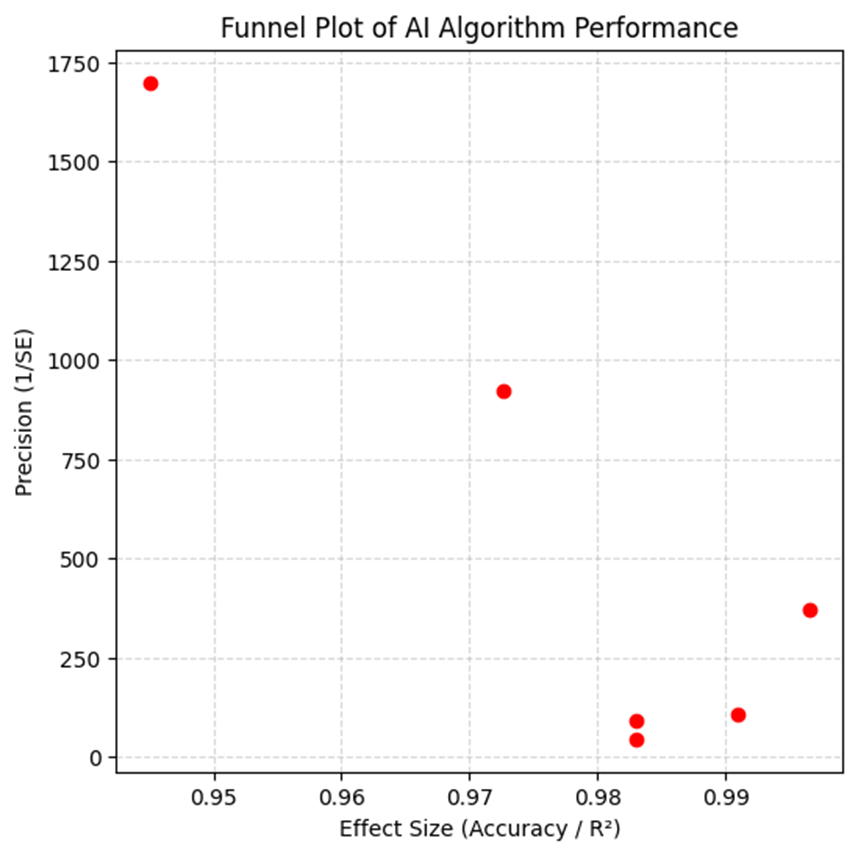
1. Introduction
The dawn of the 21st century has witnessed an unprecedented transformation in biotechnology, moving far beyond the simplistic reading of genetic codes to the active design, editing, and engineering of biological systems to address pressing global challenges. Central to this shift is the exploration of biological dark matter, a term used to describe the vast majority of microorganisms that remain uncultured in conventional laboratory settings (Handelsman, 2004). These uncultured microbes, which constitute more than 99% of microbial diversity, harbor extraordinary metabolic potential that is largely untapped by traditional methods (Alam et al., 2021). Unlocking this potential requires culture-independent, high-resolution approaches such as metagenomics, which offer function-based, sequencing-based, and single-cell strategies to identify novel natural products and enzymes capable of revolutionizing drug discovery, biofuel production, and industrial biocatalysis (Handelsman, 2004).
Function-based metagenomics enables researchers to clone environmental DNA into expression vectors and screen for specific biochemical activities in heterologous hosts. This methodology stands out because it does not rely on prior knowledge of gene sequences, allowing the discovery of entirely new genes and enzymatic pathways with unknown functions (Gillespie et al., 2002). Sequencing-based metagenomics, on the other hand, harnesses next-generation sequencing technologies and sophisticated bioinformatic platforms to detect and analyze biosynthetic gene clusters, predicting the chemical structures of previously unidentified metabolites (Alam et al., 2021). Complementing these approaches, single-cell metagenomics isolates individual genomes from complex microbial communities, providing precise taxonomic assignments and direct links between metabolic functions and specific organisms (Handelsman, 2004). Together, these strategies allow researchers to chart the uncharted microbial universe, akin to a biological deep-sea sonar mapping the vast, invisible ocean of microbial diversity.
The exploration of microbial dark matter has already yielded remarkable discoveries. Compounds such as turbomycins, fasamycins, and terragines have emerged from metagenomic libraries, while specialized metabolites including isocyanides and cadasides have demonstrated potent activity against multidrug-resistant pathogens (Gillespie et al., 2002; Feng et al., 2012). Symbiotic marine microorganisms have produced antitumor agents such as patellamide D, ascidiacyclamide, bryostatin, pederin, and onnamide, illustrating the untapped pharmacological potential of uncultured microbes (Hildebrand et al., 2004). These successes underscore the transformative role of metagenomics as both a discovery platform and a predictive framework for therapeutic innovation (Alam et al., 2021).
Parallel to microbial exploration, the concept of genomic dark matter extends to human genetics. Endogenous retroviruses, which comprise roughly 8% of the human genome, were long considered evolutionary relics but are now recognized as crucial regulators of immune function and potential biomarkers for cancer prognosis and immunotherapy (Felley-Bosco, 2023). The integration of proteogenomics—linking DNA and RNA variations with actual protein expression—enhances understanding of functional phenotypes in diseases such as colorectal cancer, enabling precision oncology approaches that anticipate drug resistance and identify novel therapeutic targets (Blank-Landeshammer et al., 2019).
The field of synthetic biology has emerged as a key enabler of these discoveries, translating genomic insights into functional applications. Saccharomyces cerevisiae, historically a model organism for fermentation, has evolved into the first eukaryote with a chemically synthesized genome, demonstrating the power of genome writing and editing (Dixon & Pretorius, 2020). Cyanobacteria have similarly been developed as photosynthetic chassis capable of converting solar energy and carbon dioxide into biofuels, high-value chemicals, and biodegradable polymers such as polyhydroxyalkanoates, aligning with broader efforts in sustainable biomanufacturing and bioenergy development (Chen et al., 2011; Cheah et al., 2018). Fast-growing strains offer high biomass productivity, positioning photosynthetic microorganisms as industrially scalable platforms for sustainable biomanufacturing (Banerjee et al., 2016).
Artificial intelligence and machine learning are increasingly integrated into these biotechnological frameworks, optimizing bioprocess parameters, predicting metabolic bottlenecks, and enabling real-time monitoring through intelligent sensing systems (Andrade Cruz et al., 2022). In microalgae-based bioprocessing, machine-learning models such as artificial neural networks and random forests have enhanced species classification, biomass prediction, and metabolic regulation, achieving high accuracies in large-scale cultivation studies (Bi et al., 2019; Ansari et al., 2021). Wearable biosensors further extend these innovations into clinical contexts by enabling continuous monitoring of biomarkers, bridging environmental biotechnology and personalized medicine (Fu et al., 2023).
The challenge of viral evolution, particularly HIV-1, illustrates the critical need for precision genomic surveillance and rapid adaptive technologies. HIV-1 exhibits extraordinary genetic diversity due to high mutation rates and frequent recombination, generating quasispecies capable of evading immune responses and antiviral therapies (Alexiev & Dimitrova, 2025). Molecular clock analyses trace cross-species transmission events from chimpanzees to humans between the 1920s and 1940s, revealing decades of viral diversification prior to the AIDS pandemic (Alexiev & Dimitrova, 2025). Modern surveillance strategies leverage next-generation sequencing to detect drug-resistance mutations and transmission networks, while biosensor arrays enable rapid, label-free detection of viral sequences (Fu et al., 2023).
The gut microbiota represents another frontier in precision biotechnology, where dysbiosis is increasingly associated with systemic disorders. Therapeutic strategies include engineered microbial consortia and targeted genetic modulation to restore microbial homeostasis and mitigate disease progression, reflecting advances in bioprocess optimization and systems-level biological control (Bagherzadeh et al., 2021; Camacho-Rodríguez et al., 2015).
Collectively, these advances exemplify a paradigm shift in biotechnology, where genomic mapping, synthetic biology, and artificial intelligence converge to transform raw biological information into actionable innovation. Machine learning–driven optimization, predictive modeling, and intelligent sensing enable adaptive, data-informed systems capable of addressing global challenges in health, sustainability, and biomanufacturing (Alrashed et al., 2018; Asnake Metekia et al., 2022). In essence, the exploration of biological and genomic dark matter heralds a new era of biotechnology—one driven by functional innovation, predictive intelligence, and the responsible harnessing of hidden biological diversity.