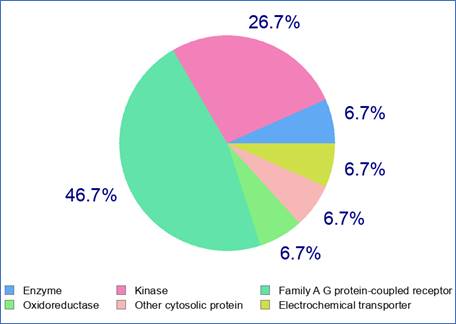
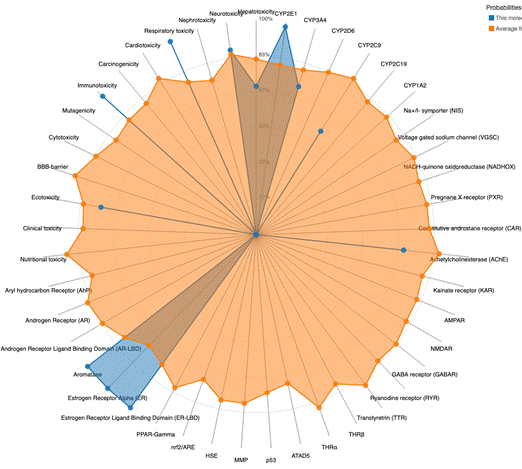
Ables, J. L., Israel, L., Wood, O., Govindarajulu, U., Fremont, R. T., Banerjee, R., Liu, H., Cohen, J., Wang, P., Kumar, K., Lu, G., DeVita, R. J., Garcia-Ocaña, A., Murrough, J. W., & Stewart, A. F. (2024). A Phase 1 single ascending dose study of pure oral harmine in healthy volunteers. Journal of psychopharmacology (Oxford, England), 38(10), 911–923. https://doi.org/10.1177/02698811241273772
Aljamali, N. M., Obaid, A. J., & Naser, M. S. (2021). Chemical Causes of Leukemia. Journal of Modern Chemistry & Chemical Technology, 12(3), 25-36p.
Alshehabi, S. (2017). Interaction between air pollution and host factors in lung cancer. The University of Manchester (United Kingdom).
Anand, U., Dey, A., Chandel, A. K. S., Sanyal, R., Mishra, A., Pandey, D. K., De Falco, V., Upadhyay, A., Kandimalla, R., Chaudhary, A., Dhanjal, J. K., Dewanjee, S., Vallamkondu, J., & Pérez de la Lastra, J. M. (2022). Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes & diseases, 10(4), 1367–1401. https://doi.org/10.1016/j.gendis.2022.02.007
Apriani, E. F., Rosana, Y., & Iskandarsyah, I. (2019). Formulation, characterization, and in vitro testing of azelaic acid ethosome-based cream against Propionibacterium acnes for the treatment of acne. Journal of advanced pharmaceutical technology & research, 10(2), 75–80. https://doi.org/10.4103/japtr.JAPTR_289_18
Arnold, M., Singh, D., Laversanne, M., Vignat, J., Vaccarella, S., Meheus, F., Cust, A. E., de Vries, E., Whiteman, D. C., & Bray, F. (2022). Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA dermatology, 158(5), 495–503. https://doi.org/10.1001/jamadermatol.2022.0160
Asgarpanah, J., & Ramezanloo, F. (2012). Chemistry, pharmacology and medicinal properties of Peganum harmala L. African Journal of pharmacy and pharmacology, 6(22), 1573-1580.
Asif Ali, M., Khan, N., Kaleem, N., Ahmad, W., Alharethi, S. H., Alharbi, B., Alhassan, H. H., Al-Enazi, M. M., Razis, A. F. A., Modu, B., Calina, D., & Sharifi-Rad, J. (2023). Anticancer properties of sulforaphane: current insights at the molecular level. Frontiers in oncology, 13, 1168321. https://doi.org/10.3389/fonc.2023.1168321
Atteya, R., Ashour, M. E., Ibrahim, E. E., Farag, M. A., & El-Khamisy, S. F. (2017). Chemical screening identifies the β-Carboline alkaloid harmine to be synergistically lethal with doxorubicin. Mechanisms of ageing and development, 161(Pt A), 141–148. https://doi.org/10.1016/j.mad.2016.04.012
Azamjah, N., Soltan-Zadeh, Y., & Zayeri, F. (2019). Global Trend of Breast Cancer Mortality Rate: A 25-Year Study. Asian Pacific journal of cancer prevention: APJCP, 20(7), 2015–2020. https://doi.org/10.31557/APJCP.2019.20.7.2015
Baldini, E., Cardarelli, S., Campese, A. F., Lori, E., Fallahi, P., Virili, C., Forte, F., Pironi, D., Di Matteo, F. M., Palumbo, P., Costanzo, M. L., D'Andrea, V., Centanni, M., Sorrenti, S., Antonelli, A., & Ulisse, S. (2024). Evaluation of the Therapeutic Effects of Harmine on Anaplastic Thyroid Cancer Cells. International journal of molecular sciences, 25(2), 1121. https://doi.org/10.3390/ijms25021121
Benny, F., Kumar, S., Jayan, J., Abdelgawad, M. A., Ghoneim, M. M., Kumar, A., Manoharan, A., Susan, R., Sudevan, S. T., & Mathew, B. (2023). Review of β-carboline and its derivatives as selective MAO-A inhibitors. Archiv der Pharmazie, 356(7), e2300091. https://doi.org/10.1002/ardp.202300091
Berrougui, H., Isabelle, M., Cloutier, M., Hmamouchi, M., & Khalil, A. (2006). Protective effects of Peganum harmala L. extract, harmine and harmaline against human low-density lipoprotein oxidation. The Journal of pharmacy and pharmacology, 58(7), 967–974. https://doi.org/10.1211/jpp.58.7.0012
Bhuia, M. S., Aktar, M. A., Chowdhury, R., Ferdous, J., Rahman, M. A., Al Hasan, M. S., & Islam, M. T. (2023a). Therapeutic potentials of ononin with mechanistic insights: A comprehensive review. Food Bioscience, 56, 103302.
Bhuia, M. S., Chowdhury, R., Sonia, F. A., Kamli, H., Shaikh, A., El-Nashar, H. A. S., El-Shazly, M., & Islam, M. T. (2023b). Anticancer Potential of the Plant-Derived Saponin Gracillin: A Comprehensive Review of Mechanistic Approaches. Chemistry & biodiversity, 20(9), e202300847. https://doi.org/10.1002/cbdv.202300847
Bizuayehu, H. M., Ahmed, K. Y., Kibret, G. D., Dadi, A. F., Belachew, S. A., Bagade, T., Tegegne, T. K., Venchiarutti, R. L., Kibret, K. T., Hailegebireal, A. H., Assefa, Y., Khan, M. N., Abajobir, A., Alene, K. A., Mengesha, Z., Erku, D., Enquobahrie, D. A., Minas, T. Z., Misgan, E., & Ross, A. G. (2024). Global Disparities of Cancer and Its Projected Burden in 2050. JAMA network open, 7(11), e2443198. https://doi.org/10.1001/jamanetworkopen.2024.43198
Brito-da-Costa, A. M., Dias-da-Silva, D., Gomes, N. G. M., Dinis-Oliveira, R. J., & Madureira-Carvalho, Á. (2020). Toxicokinetics and Toxicodynamics of Ayahuasca Alkaloids N, N-Dimethyltryptamine (DMT), Harmine, Harmaline and Tetrahydroharmine: Clinical and Forensic Impact. Pharmaceuticals (Basel, Switzerland), 13(11), 334. https://doi.org/10.3390/ph13110334
Chen, Q., Chao, R., Chen, H., Hou, X., Yan, H., Zhou, S., Peng, W., & Xu, A. (2005). Antitumor and neurotoxic effects of novel harmine derivatives and structure-activity relationship analysis. International journal of cancer, 114(5), 675–682. https://doi.org/10.1002/ijc.20703
Chen, Z. Y., Li, J., Zhu, S. D., Li, Z. D., Yu, J. L., Wu, J., ... & Zeng, L. H. (2022). Harmine reinforces the effects of regorafenib on suppressing cell proliferation and inducing apoptosis in liver cancer cells. Experimental and Therapeutic Medicine, 23(3), 1-9.
Chin, L. T., Liu, K. W., Chen, Y. H., Hsu, S. C., & Huang, L. (2021). Cell-based assays and molecular simulation reveal that the anti-cancer harmine is a specific matrix metalloproteinase-3 (MMP-3) inhibitor. Computational biology and chemistry, 94, 107556. https://doi.org/10.1016/j.compbiolchem.2021.107556
Chamberlain, M. C., Baik, C. S., Gadi, V. K., Bhatia, S., & Chow, L. Q. M. (2017). Systemic therapy of brain metastases: non–small cell lung cancer, breast cancer, and melanoma. Neuro-Oncology, 19(1), i1–i24. https://doi.org/10.1093/neuonc/now197
Chuang, S. C., La Vecchia, C., & Boffetta, P. (2009). Liver cancer: descriptive epidemiology and risk factors other than HBV and HCV infection. Cancer letters, 286(1), 9-14.
Dana, E. D., Garcia De Lomas, J., & Sanchez, J. (2010). Effects of the aqueous extracts of Zygophyllum fabago on the growth of Fusarium oxyosporum f. sp. melonis and Pythium aphanidermatum. Weed biology and management, 10(3), 170-175.
de Frias, U. A., Costa, M. C. M., Takahashi, J. A., & Oki, Y. (2012). Banisteriopsis Species: A source of bioactive of potential medical application. Int. J. Biotechnol. Wellness Ind, 1, 163-171.
de Groot, P., & Munden, R. F. (2012). Lung cancer epidemiology, risk factors, and prevention. Radiologic Clinics, 50(5), 863-876.
Devi, G., Sudhakar, K., Vasupradaa, A. P., Sravya, V., Manasa, V., & Yasaswini, E. (2021). Medicinal plants in india and it’s antioxidant potential–a review. Revista geintec-gestao inovacao e tecnologias, 11(4), 1397-1405.
Ding, Y., He, J., Huang, J., Yu, T., Shi, X., Zhang, T., Yan, G., Chen, S., & Peng, C. (2019). Harmine induces anticancer activity in breast cancer cells via targeting TAZ. International journal of oncology, 54(6), 1995–2004. https://doi.org/10.3892/ijo.2019.4777
Dongdong Z, Jin Y, Yang T, Yang Q, Wu B, Chen Y, Luo Z, Liang L, Liu Y, Xu A, Tong X, Can C, Ding L, Tu H, Tan Y, Jiang H, Liu X, Shen H, Liu L, Pan Y, Wei Y and Zhou F (2019) Antiproliferative and Immunoregulatory Effects of Azelaic Acid Against Acute Myeloid Leukemia via the Activation of Notch Signaling Pathway. Front. Pharmacol. 10:1396. doi: 10.3389/fphar.2019.01396
Doostmohammadi, A., Jooya, H., Ghorbanian, K., Gohari, S., & Dadashpour, M. (2024). Potentials and future perspectives of multi-target drugs in cancer treatment: the next generation anti-cancer agents. Cell Communication and Signaling, 22(1), 228. https://doi.org/10.1186/s12964-024-01607-9
Dorato, M. A., & Buckley, L. A. (2006). Toxicology in the drug discovery and development process. Current protocols in pharmacology, 32(1), 10-3.
Dos Santos, R. G., & Hallak, J. E. (2017). Effects of the natural β-carboline alkaloid harmine, a main constituent of ayahuasca, in memory and in the hippocampus: A systematic literature review of preclinical studies. Journal of psychoactive drugs, 49(1), 1-10.
El Fissi, H., Ameziane, H., Bouzid, F., Achqra, I., Msanda, F., & Alif, N. (2024). Isolation, digital analysis, and semi-quantification of harmaline and harmine from Peganum harmala L. Euro-Mediterranean Journal for Environmental Integration, 1-6.
Elumalai, P., Muninathan, N., Megalatha, S. T., Suresh, A., Kumar, K. S., Jhansi, N., Kalaivani, K., & Krishnamoorthy, G. (2022). An Insight into Anticancer Effect of Propolis and Its Constituents: A Review of Molecular Mechanisms. Evidence-based complementary and alternative medicine: eCAM, 2022, 5901191. https://doi.org/10.1155/2022/5901191
Fahr, A., Hoogevest, P. van, May, S., Bergstrand, N., & S. Leigh, M. L. (2005). Transfer of lipophilic drugs between liposomal membranes and biological interfaces: Consequences for drug delivery. European Journal of Pharmaceutical Sciences, 26(3–4), 251–265. https://doi.org/10.1016/j.ejps.2005.05.012
Filali, I., Bouajila, J., Znati, M., Bousejra-El Garah, F., & Ben Jannet, H. (2015). Synthesis of new isoxazoline derivatives from harmine and evaluation of their anti-Alzheimer, anti-cancer and anti-inflammatory activities. Journal of enzyme inhibition and medicinal chemistry, 30(3), 371-376.
Frecska, E., Bokor, P., & Winkelman, M. (2016). The therapeutic potentials of ayahuasca: possible effects against various diseases of civilization. Frontiers in pharmacology, 7, 35.
Friedman, L. M., Furberg, C. D., DeMets, D. L., Reboussin, D. M., & Granger, C. B. (2015). Fundamentals of clinical trials. springer.
Friedman, L. M., Furberg, C. D., DeMets, D. L., Reboussin, D. M., & Granger, C. B. (2015). Fundamentals of clinical trials. springer.
Frison, G., Favretto, D., Zancanaro, F., Fazzin, G., & Ferrara, S. D. (2008). A case of β-carboline alkaloid intoxication following ingestion of Peganum harmala seed extract. Forensic Science International, 179(2-3), e37-e43.
Frye, A., & Haustein, C. (2007). Extraction, identification, and quantification of harmala alkaloids in three species of Passiflora. American journal of undergraduate research, 6(3), 19-26.
Gandomani, H. S., Aghajani, M., Mohammadian-Hafshejani, A., Tarazoj, A. A., Pouyesh, V., & Salehiniya, H. (2017). Colorectal cancer in the world: incidence, mortality and risk factors. Biomedical Research and Therapy, 4(10), 1656-1675.
Gao, J., Zhu, H., Wan, H., Zou, X., Ma, X., & Gao, G. (2017). Harmine suppresses the proliferation and migration of human ovarian cancer cells through inhibiting ERK/CREB pathway. Oncology reports, 38(5), 2927–2934. https://doi.org/10.3892/or.2017.5952
Gupta, N., & Qanungo, K. (2023, May). Cigarette smoking and its association with lung cancer. In AIP Conference Proceedings (Vol. 2535, No. 1). AIP Publishing.
Hashemi Sheikh Shabani, S., Seyed Hasan Tehrani, S., Rabiei, Z., Tahmasebi Enferadi, S., & Vannozzi, G. P. (2015). Peganum harmala L.'s anti-growth effect on a breast cancer cell line. Biotechnology reports (Amsterdam, Netherlands), 8, 138–143. https://doi.org/10.1016/j.btre.2015.08.007
He, J., Chen, S., Yu, T., Chen, W., Huang, J., Peng, C., & Ding, Y. (2022). Harmine suppresses breast cancer cell migration and invasion by regulating TAZ-mediated epithelial-mesenchymal transition. American journal of cancer research, 12(6), 2612–2626.
Herraiz, T., González, D., Ancín-Azpilicueta, C., Arán, V. J., & Guillén, H. (2010). β-Carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO). Food and Chemical Toxicology, 48(3), 839-845.
Huang, M., Lu, J. J., & Ding, J. (2021). Natural products in cancer therapy: Past, present and future. Natural products and bioprospecting, 11(1), 5-13.
Islam, M. T., & Dantas, R. C. M. (2013). Anticancer prospection of salicin, a historical origin of aspirin. Nutr Cancer, 65, 1045-1058.
Javeed, M., Rasul, A., Hussain, G., Jabeen, F., Rasool, B., Shafiq, N., ... & Ali, M. (2018). Harmine and its derivatives: Biological activities and therapeutic potential in human diseases. ||| Bangladesh Journal of Pharmacology|||, 13(3), 203-213.
Javeed, M., Rasul, A., Hussain, G., Jabeen, F., Rasool, B., Shafiq, N., ... & Ali, M. (2018). Harmine and its derivatives: Biological activities and therapeutic potential in human diseases. ||| Bangladesh Journal of Pharmacology|||, 13(3), 203-213.
Jebar, A. H., Errington-Mais, F., Vile, R. G., Selby, P. J., Melcher, A. A., & Griffin, S. (2015). Progress in clinical oncolytic virus-based therapy for hepatocellular carcinoma. Journal of General Virology, 96(Pt_7), 1533-1550.
K. Addo-Boadu; J. Wojta; G. Christ; P. Hufnagl; H. Pehamberger; B.R. Binder. (1996). Azelaic acid decreases the fibrinolytic potential of cultured human melanoma cells in vitro., 103(2), 0–129. doi:10.1016/0304-3835(96)04185-7.
Kadyan, P., & Singh, L. (2024). Unraveling the mechanistic interplay of mediators orchestrating the neuroprotective potential of harmine. Pharmacological Reports, 1-14.
Kartal, M. U. R. A. T., Altun, M. L., & Kurucu, S. (2003). HPLC method for the analysis of harmol, harmalol, harmine and harmaline in the seeds of Peganum harmala L. Journal of pharmaceutical and biomedical analysis, 31(2), 263-269.
Kciuk, M., Marciniak, B., & Kontek, R. (2020). Irinotecan-Still an Important Player in Cancer Chemotherapy: A Comprehensive Overview. International journal of molecular sciences, 21(14), 4919. https://doi.org/10.3390/ijms21144919
Khairudin, N.; Basri, M.; Fard Masoumi, H.R.; Samson, S.; Ashari, S.E. Enhancing the Bioconversion of Azelaic Acid to Its Derivatives by Response Surface Methodology. Molecules 2018, 23, 397. https://doi.org/10.3390/molecules23020397.
Khan, H., & Nabavi, S. M. (2019). Passiflora (Passiflora incarnata). In Nonvitamin and nonmineral nutritional supplements (pp. 361-366). Academic Press.
Kleeff, J., Korc, M., Apte, M., La Vecchia, C., Johnson, C. D., Biankin, A. V., ... & Neoptolemos, J. P. (2016). Pancreatic cancer. Nature reviews Disease primers, 2(1), 1-22.
Kluska, M., & Wozniak, K. (2021). Natural Polyphenols as Modulators of Etoposide Anti-Cancer Activity. International journal of molecular sciences, 22(12), 6602. https://doi.org/10.3390/ijms22126602
Kruger, E., Toraih, E. A., Hussein, M. H., Shehata, S. A., Waheed, A., Fawzy, M. S., & Kandil, E. (2022). Thyroid carcinoma: a review for 25 years of environmental risk factors studies. Cancers, 14(24), 6172.
Kumar, A. (2022). Plant alkaloids. In Phytoconstituents and Antifungals (pp. 53-64). Academic Press.
Kumar, K., Wang, P., Sanchez, R., Swartz, E. A., Stewart, A. F., & DeVita, R. J. (2018). Development of kinase-selective, harmine-based DYRK1A inhibitors that induce pancreatic human β-cell proliferation. Journal of medicinal chemistry, 61(17), 7687-7699.
Lewerenz, L., Hijazin, T., Abouzeid, S., Hänsch, R., & Selmar, D. (2020). Pilot study on the uptake and modification of harmaline in acceptor plants: An innovative approach to visualize the interspecific transfer of natural products. Phytochemistry, 174, 112362. https://doi.org/10.1016/j.phytochem.2020.112362
Li, C., Wang, Y., Wang, C., Yi, X., Li, M., & He, X. (2017). Anticancer activities of harmine by inducing a pro-death autophagy and apoptosis in human gastric cancer cells. Phytomedicine: international journal of phytotherapy and phytopharmacology, 28, 10–18. https://doi.org/10.1016/j.phymed.2017.02.008
Li, Z., Chen, L., He, C., Han, Y., Han, M., Zhang, Y., Qi, L., Xing, X., Huang, W., Gao, Z., & Xing, J. (2020). Improving anti-tumor outcomes for colorectal cancer therapy through in situ thermosensitive gel loading harmine. American journal of translational research, 12(5), 1658–1671.
Liao, Y., Wu, X., Luo, W., Chen, J., Huang, Y., Ma, K., ... & Wang, X. (2023). Azelaic Acid Regulates the Renin–Angiotensin System and Improves Colitis Based on Network Pharmacology and Experimentation. ACS omega, 8(17), 15217-15228. doi.org/10.1021/acsomega.3c00210.
Linares, M. A., Zakaria, A., & Nizran, P. (2015). Skin cancer. Prim Care, 42(4), 645-659.
Liu, J., Li, Q., Liu, Z., Lin, L., Zhang, X., Cao, M., & Jiang, J. (2016). Harmine induces cell cycle arrest and mitochondrial pathway-mediated cellular apoptosis in SW620 cells via inhibition of the Akt and ERK signaling pathways. Oncology reports, 35(6), 3363–3370. https://doi.org/10.3892/or.2016.4695
Liu, X., Li, M., Tan, S., Wang, C., Fan, S., & Huang, C. (2017). Harmine is an inflammatory inhibitor through the suppression of NF-κB signaling. Biochemical and biophysical research communications, 489(3), 332–338. https://doi.org/10.1016/j.bbrc.2017.05.126
Louis, D. N. (2016). WHO classification of tumours of the central nervous system. (No Title).
Lupan, I., Bintintan, V., Deleanu, D., & Samasca, G. (2024). Epigenetic Regulation of DNA Methylation and RNA Interference in Gastric Cancer: A 2024 Update. Biomedicines, 12(9), 2001. https://doi.org/10.3390/biomedicines12092001
Ma, Y., & Wink, M. (2010). The beta-carboline alkaloid harmine inhibits BCRP and can reverse resistance to the anticancer drugs mitoxantrone and camptothecin in breast cancer cells. Phytotherapy research: PTR, 24(1), 146–149. https://doi.org/10.1002/ptr.2860
Malik, Deepinder Singh; Kaur, Gurpreet. (2019). Exploring therapeutic potential of azelaic acid loaded NLCs for the treatment of acne vulgaris. Journal of Drug Delivery Science and Technology, (), 101418–. doi: 10.1016/j.jddst.2019.101418.
Mancebo, S. E., & Wang, S. Q. (2014). Skin cancer: role of ultraviolet radiation in carcinogenesis. Reviews on environmental health, 29(3), 265-273.
Manosroi, A., Panyosak, A., Rojanasakul, Y., & Manosroi, J. (2007). Characteristics and anti-proliferative activity of azelaic acid and its derivatives entrapped in bilayer vesicles in cancer cell lines. Journal of Drug Targeting, 15(5), 334–341. https://doi.org/10.1080/10611860701349315
Mastrofrancesco, A., Ottaviani, M., Aspite, N., Cardinali, G., Izzo, E., Graupe, K., Zouboulis, C. C., Camera, E., & Picardo, M. (2010). Azelaic acid modulates the inflammatory response in normal human keratinocytes through PPARgamma activation. Experimental dermatology, 19(9), 813–820. https://doi.org/10.1111/j.1600-0625.2010.01107.x
Mayad, E. H., Basaid, K., Furze, J. N., Heimeur, N., Senhaji, B., Chebli, B., ... & Ferji, Z. (2019). Reversible nematostatic effect of Peganum harmala L.(Nitrariaceae) on Meloidogyne javanica.
McKenna, D. J., Towers, G. N., & Abbott, F. (1984). Monoamine oxidase inhibitors in South American hallucinogenic plants: tryptamine and β-carboline constituents of ayahuasca. Journal of ethnopharmacology, 10(2), 195-223.
Meyer, E. A., Castellano, R. K., & Diederich, F. (2003). Interactions with Aromatic Rings in Chemical and Biological Recognition. Angewandte Chemie International Edition, 42(11), 1210–1250. https://doi.org/10.1002/anie.200390319
Moloudizargari, M., Mikaili, P., Aghajanshakeri, S., Asghari, M. H., & Shayegh, J. (2013). Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacognosy reviews, 7(14), 199–212. https://doi.org/10.4103/0973-7847.120524
Morales-García, J. A., De la Fuente Revenga, M., Alonso-Gil, S., Rodríguez-Franco, M. I., Feilding, A., Perez-Castillo, A., & Riba, J. (2017). The alkaloids of Banisteriopsis caapi, the plant source of the Amazonian hallucinogen Ayahuasca, stimulate adult neurogenesis in vitro. Scientific reports, 7(1), 5309.
Morsy, M. H. E., Nabil, Z. I., Darwish, S. T., Al-Eisa, R. A., & Mehana, A. E. (2023). Anti-Diabetic and Anti-Adipogenic Effect of Harmine in High-Fat-Diet-Induced Diabetes in Mice. Life (Basel, Switzerland), 13(8), 1693. https://doi.org/10.3390/life13081693
M, S., N, R. P., Chakraborty, A., & Rajendrasozhan, S. (2022). Proteomic profiling of Deinococcus radiodurans with response to thioredoxin reductase inhibitor and ionizing radiation treatment. Journal of Proteomics, 267, 104697. https://doi.org/10.1016/j.jprot.2022.104697
Muniraj, T., Jamidar, P. A., & Aslanian, H. R. (2013). Pancreatic cancer: a comprehensive review and update. Disease-a-month, 59(11), 368-402.
Mvondo, J. G. M., Matondo, A., Mawete, D. T., Bambi, S.-M. N., Mbala, B. M., & Lohohola, P. O. (2021). In Silico ADME/T Properties of Quinine Derivatives using SwissADME and pkCSM Webservers. International Journal of TROPICAL DISEASE & Health, 1–12. https://doi.org/10.9734/ijtdh/2021/v42i1130492
Nafie, E., Lolarga, J., Lam, B., Guo, J., Abdollahzadeh, E., Rodriguez, S., Glackin, C., & Liu, J. (2021). Harmine inhibits breast cancer cell migration and invasion by inducing the degradation of Twist1. PloS one, 16(2), e0247652. https://doi.org/10.1371/journal.pone.0247652
Nassiri-Asl, M., Shariati-Rad, S., & Zamansoltani, F. (2007). Anticonvulsant effects of aerial parts of Passiflora incarnata extract in mice: involvement of benzodiazepine and opioid receptors. BMC complementary and alternative medicine, 7, 1-6.
Nikam, T. D., Ebrahimi, M. A., & Patil, V. A. (2009). Embryogenic callus culture of Tribulus terrestris L. a potential source of harmaline, harmine and diosgenin. Plant Biotechnology Reports, 3, 243-250.
Ock, C. W., & Kim, G. D. (2021). Harmine Hydrochloride Mediates the Induction of G2/M Cell Cycle Arrest in Breast Cancer Cells by Regulating the MAPKs and AKT/FOXO3a Signaling Pathways. Molecules, 26(21), 6714. https://doi.org/10.3390/molecules26216714
Ohgaki, H., & Kleihues, P. (2005). Epidemiology and etiology of gliomas. Acta neuropathologica, 109, 93-108.
Oodi, A., Norouzi, H., Amirizadeh, N., Nikougoftar, M., & Vafaie, Z. (2017). Harmine, a Novel DNA Methyltransferase 1 Inhibitor in the Leukemia Cell Line. Indian journal of hematology & blood transfusion: an official journal of Indian Society of Hematology and Blood Transfusion, 33(4), 509–515. https://doi.org/10.1007/s12288-016-0770-z
Pan Y, Liu D, Wei Y, Su D, Lu C, Hu Y and Zhou F (2017) Azelaic Acid Exerts Antileukemic Activity in Acute Myeloid Leukemia. Front. Pharmacol. 8:359. doi: 10.3389/fphar.2017.00359.
Park, W., Chawla, A., & O’Reilly, E. M. (2021). Pancreatic cancer: a review. Jama, 326(9), 851-862.
Patel, K., Gadewar, M., Tripathi, R., Prasad, S. K., & Patel, D. K. (2012). A review on medicinal importance, pharmacological activity and bioanalytical aspects of beta-carboline alkaloid “Harmine”. Asian Pacific journal of tropical biomedicine, 2(8), 660-664.
Pecorelli, S., Odicino, F., & Favalli, G. (2002). Interval debulking surgery in advanced epithelial ovarian cancer. Best Practice & Research Clinical Obstetrics & Gynaecology, 16(4), 573-583.
Pfau, W., & Skog, K. (2004). Exposure to β-carbolines norharman and harman. Journal of Chromatography B, 802(1), 115-126.
Piantadosi, S. (2024). Clinical trials: a methodologic perspective. John Wiley & Sons.
Piazuelo, M. B., & Correa, P. (2013). Gastric cancer: overview. Colombia Medica, 44(3), 192-201.
Pokhriyal, R., Hariprasad, R., Kumar, L., & Hariprasad, G. (2019). Chemotherapy resistance in advanced ovarian cancer patients. Biomarkers in cancer, 11, 1179299X19860815.
Prinsloo, D. (2019). An investigation of plant derived compounds as inhibitors of monoamine oxidase (Doctoral dissertation, North-West University (South-Africa). Potchefstroom Campus).
Qadir, S. U., & Raja, V. (2021). Herbal medicine: Old practice and modern perspectives. In Phytomedicine (pp. 149-180). Academic Press.
Rahimi Darehbagh, R., & Rezaei, N. (2024). Introduction to Stem Cell Therapy and Blood Cancers. In Hematological Cancer Diagnosis and Treatment: An Interdisciplinary Approach (pp. 1-15). Cham: Springer Nature Switzerland.
raji F, Sadeghinia A, Shahmoradi Z, Siadat AH, Jooya A. Efficacy of topical azelaic acid gel in the treatment of mild-moderate acne vulgaris. Indian J Dermatol Venereol Leprol 2007;73:94-96.
Rasool, S., Kadla, S. A., Rasool, V., & Ganai, B. A. (2013). A comparative overview of general risk factors associated with the incidence of colorectal cancer. Tumor Biology, 34, 2469-2476.
Rawla, P., & Barsouk, A. (2019). Epidemiology of gastric cancer: global trends, risk factors and prevention. Gastroenterology Review/Przeglad Gastroenterologiczny, 14(1), 26-38.
Remko, M., Bohác, A., & Kováciková, L. (2011). Molecular structure, pKa, lipophilicity, solubility, absorption, polar surface area, and blood brain barrier penetration of some antiangiogenic agents. Structural Chemistry, 22(3), 635–648. https://doi.org/10.1007/s11224-011-9741-z
Rooth, C. (2013). Ovarian cancer: risk factors, treatment and management. British journal of nursing, 22(Sup17), S23-S30.
Ruan, S., Jia, F., & Li, J. (2017). Potential Antitumor Effect of Harmine in the Treatment of Thyroid Cancer. Evidence-based complementary and alternative medicine: eCAM, 2017, 9402615. https://doi.org/10.1155/2017/9402615
Salehi, F., Dunfield, L., Phillips, K. P., Krewski, D., & Vanderhyden, B. C. (2008). Risk factors for ovarian cancer: an overview with emphasis on hormonal factors. Journal of Toxicology and Environmental Health, Part B, 11(3-4), 301-321.
Sharifi, S., Sattari, T. N., Zebarjadi, A., Majd, A., & Ghasempour, H. (2014). The influence of Agrobacterium rhizogenes on induction of hairy roots and ß-carboline alkaloids production in Tribulus terrestris L. Physiology and molecular biology of plants, 20, 69-80.
Sharma, R., Kumar, S., Kaushik, N., & Singh, B. (2025). Decoding the Mystery of Blood Cancer: Cause, Diagnosis, and Management. Current Cancer Therapy Reviews, 21(1), 40-53.
Sharmaa, Neha, et al. "Azelaic acid attenuatesrotenone-induced behavioural alterations in parkinson’s disease rat model." Plant Arch 21 (2021): 2333-2337. https://doi.org/10.51470/PLANTARCHIVES.2021.v21.S1.383.
Shinji, S., Yamada, T., Matsuda, A., Sonoda, H., Ohta, R., Iwai, T., ... & Yoshida, H. (2022). Recent advances in the treatment of colorectal cancer: a review. Journal of Nippon Medical School, 89(3), 246-254.
Shtivelman, E., Hensing, T., Simon, G. R., Dennis, P. A., Otterson, G. A., Bueno, R., & Salgia, R. (2014). Molecular pathways and therapeutic targets in lung cancer. Oncotarget, 5(6), 1392.
Silaghi, H., Lozovanu, V., Georgescu, C. E., Pop, C., Nasui, B. A., Catoi, A. F., & Silaghi, C. A. (2022). State of the art in the current management and future directions of targeted therapy for differentiated thyroid cancer. International Journal of Molecular Sciences, 23(7), 3470.
Škubník, J., Pavlícková, V. S., Ruml, T., & Rimpelová, S. (2021). Vincristine in combination therapy of cancer: emerging trends in clinics. Biology, 10(9), 849.
SRS Mota, N., R. Kviecinski, M., B. Felipe, K., MAS Grinevicius, V., Siminski, T., M. Almeida, G., ... & C. Pedrosa, R. (2020). β-carboline alkaloid harmine induces DNA damage and triggers apoptosis by a mitochondrial pathway: study in silico, in vitro and in vivo. International Journal of Functional Nutrition, 1(1), 1.
Stupp, R., Mason, W. P., Van Den Bent, M. J., Weller, M., Fisher, B., Taphoorn, M. J., ... & Mirimanoff, R. O. (2005). Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New England journal of medicine, 352(10), 987-996.
Sukhachev, V. S., Dmitriev, A. V., Ivanov, S. M., Savosina, P. I., Druzhilovskiy, D. S., Filimonov, D. A., & Poroikov, V. V. (2024). Assessment of the Efficiency of Selecting Promising Compounds During Virtual Screening Based on Various Estimations of Drug-Likeness. Pharmaceutical Chemistry Journal, 58(9), 1388–1396. https://doi.org/10.1007/s11094-025-03286-3
Sun, K., Tang, X. H., & Xie, Y. K. (2015). Paclitaxel combined with harmine inhibits the migration and invasion of gastric cancer cells through downregulation of cyclooxygenase-2 expression. Oncology letters, 10(3), 1649–1654. https://doi.org/10.3892/ol.2015.3425
Sun, P., Zhang, S., Li, Y., & Wang, L. (2014). Harmine mediated neuroprotection via evaluation of glutamate transporter 1 in a rat model of global cerebral ischemia. Neuroscience letters, 583, 32–36. https://doi.org/10.1016/j.neulet.2014.09.023
Sun, Y. S., Zhao, Z., Yang, Z. N., Xu, F., Lu, H. J., Zhu, Z. Y., Shi, W., Jiang, J., Yao, P. P., & Zhu, H. P. (2017). Risk Factors and Preventions of Breast Cancer. International journal of biological sciences, 13(11), 1387–1397. https://doi.org/10.7150/ijbs.21635
Tang, X., Kurban, M., Hafiz, I., Shen, Q., & Wang, M. (2023). Preparation of hyaluronic acid-loaded Harmine polymeric micelles and in vitro effect anti-breast cancer. European journal of pharmaceutical sciences: official journal of the European Federation for Pharmaceutical Sciences, 183, 106388. https://doi.org/10.1016/j.ejps.2023.106388
Timbilla, A. A., Vrabec, R., Havelek, R., Rezacova, M., Chlebek, J., Blunden, G., & Cahlikova, L. (2024). The anticancer properties of harmine and its derivatives. Phytochemistry Reviews, 1-30.
Tiwari, S., Singh, S., Tripathi, S., & Kumar, S. (2015). A pharmacological review: Passiflora species. Asian Journal of Pharmaceutical Research, 5(4), 195-202.
Tsuda, H., Ohshima, Y., Nomoto, H., Fujita, K., Matsuda, E., Iigo, M., Takasuka, N., & Moore, M. A. (2004). Cancer prevention by natural compounds. Drug metabolism and pharmacokinetics, 19(4), 245–263. https://doi.org/10.2133/dmpk.19.245
Wang, J., & Wu, S. G. (2023). Breast Cancer: An Overview of Current Therapeutic Strategies, Challenge, and Perspectives. Breast cancer (Dove Medical Press), 15, 721–730. https://doi.org/10.2147/BCTT.S432526
Wu, L. W., Zhang, J. K., Rao, M., Zhang, Z. Y., Zhu, H. J., & Zhang, C. (2019). Harmine suppresses the proliferation of pancreatic cancer cells and sensitizes pancreatic cancer to gemcitabine treatment. OncoTargets and therapy, 12, 4585–4593. https://doi.org/10.2147/OTT.S205097
Xing, M. (2013). Molecular pathogenesis and mechanisms of thyroid cancer. Nature Reviews Cancer, 13(3), 184-199.
Yu, X. J., Sun, K., Tang, X. H., Zhou, C. J., Sun, H., Yan, Z., Fang, L., Wu, H. W., Xie, Y. K., & Gu, B. (2016). Harmine combined with paclitaxel inhibits tumor proliferation and induces apoptosis through down-regulation of cyclooxygenase-2 expression in gastric cancer. Oncology letters, 12(2), 983–988. https://doi.org/10.3892/ol.2016.4696
Zhang, H., Sun, K., Ding, J., Xu, H., Zhu, L., Zhang, K., Li, X., & Sun, W. (2014). Harmine induces apoptosis and inhibits tumor cell proliferation, migration and invasion through down-regulation of cyclooxygenase-2 expression in gastric cancer. Phytomedicine: international journal of phytotherapy and phytopharmacology, 21(3), 348–355. https://doi.org/10.1016/j.phymed.2013.09.007
Zhang, S., Cheng, C., Lin, Z., Xiao, L., Su, X., Zheng, L., ... & Liu, J. (2022). The global burden and associated factors of ovarian cancer in 1990–2019: findings from the Global Burden of Disease Study 2019. BMC Public Health, 22(1), 1455.
Zhao, L., & Wink, M. (2013). The β-carboline alkaloid harmine inhibits telomerase activity of MCF-7 cells by down-regulating hTERT mRNA expression accompanied by an accelerated senescent phenotype. PeerJ, 1, e174. https://doi.org/10.7717/peerj.174Bei, Y. Y., Yuan, Z. Q., Zhang, L., Zhou, X. F., Chen, W. L., Xia, P., Liu, Y., You, B. G., Hu, X. J., Zhu, Q. L., Zhang, C. G., Zhang, X. N., & Jin, Y. (2014). No vel self-assembled micelles based on palmitoyl-trimethyl-chitosan for efficient delivery of harmine to liver cancer. Expert opinion on drug delivery, 11(6), 843–854. https://doi.org/10.1517/17425247.2014.893292
Zhao, T., He, Y., Wang, J., Ding, K., Wang, C., & Wang, Z. (2011). Inhibition of Human Cytochrome P450 Enzymes 3A4 and 2D6 by β-Carboline Alkaloids, Harmine Derivatives. Phytotherapy Research, 25(11), 1671–1677. https://doi.org/10.1002/ptr.3458
Zhong, F., Chen, Y., Chen, J., Liao, H., Li, Y., & Ma, Y. (2022). Jatrorrhizine: a review of sources, pharmacology, pharmacokinetics and toxicity. Frontiers in Pharmacology, 12, 783127.
Zhu, J., Zhu, H., Zhu, Q., Xu, S. L., Xiao, L., Zhang, M. Y., & Gao, J. (2024). The roles of autophagy, ferroptosis and pyroptosis in the anti-ovarian cancer mechanism of harmine and their crosstalk. Scientific reports, 14(1), 6504. https://doi.org/10.1038/s41598-024-57196-7